Search the whole station Crushing Equipment
Lead zinc flotation process is the most widely used in separating sulfide ore , to separate zinc and plumbum, and sometimes for separating sulfide iron and other minerals.
Efficient lead-zinc separation comes from knowing the ore, controlling grinding, and using precise flotation steps. Need the right recipe of chemicals and conditions to float lead first, then zinc.
Understanding specific ore type is the very first step. It guides everything that comes after.
Ore’s minerals (like galena, sphalerite, pyrite) type and how they are locked together (texture, grain size) control the whole processing plan. This includes how fine to grind and how to float the minerals.



Need to look closely at the ore before ZONEDING designs the plant. Process mineralogy tells what minerals are present and how they exist together. This is more important than just knowing the head grade. Many projects fail because they don’t study this enough early on.
The main lead mineral is usually galena (PbS). The main zinc mineral is sphalerite (ZnS). Need to know about other minerals present. Pyrite (FeS₂) is very common and can interfere with flotation. Gangue minerals like quartz, calcite, or dolomite make up the rest of the rock. Knowing the types and amounts of all minerals helps choose the right processing steps. For example, sphalerite can contain different amounts of iron. High-iron sphalerite (marmatite) floats differently than low-iron sphalerite.
Minerals are often locked together. Need to grind the ore fine enough to free the valuable lead and zinc minerals from the waste minerals and from each other. This is called liberation. Process mineralogy studies tell us the size at which most galena and sphalerite particles become liberated. This size becomes the target for the grinding circuit. Grinding too coarse means poor liberation and poor recovery. Grinding too fine (over-grinding) creates slimes, wastes energy, and makes flotation difficult. Finding the best grind size is a balance.
The non-valuable minerals (gangue) also affect processing. Some gangue minerals, like certain clays or talc, can float unintentionally, lowering concentrate grade. Others, like carbonates (calcite), consume acid if leaching is considered. Pyrite is often the most troublesome gangue sulfide. It tends to float easily and needs to be suppressed. Detailed mineralogy work upfront saves a lot of money and headaches later.
The best way is usually staged Crushing. Then use grinding mills like a Ball Mill or Rod Mill. You aim for the liberation size found in your ore study. Avoid over-grinding because it creates slimes that hurt flotation.


Crushing and grinding are the first steps to prepare the ore for separation. The main goal is liberation – freeing the valuable lead and zinc minerals from the gangue and each other. But must do this carefully.
As explained before, the target grind size comes from studying the ore’s mineralogy and texture. Break the rock just enough to liberate the minerals. Donot want to create excessive fine particles (slimes). Slimes have a huge surface area. They consume a lot of expensive reagents. They also make flotation difficult to control. Finding the economic optimum grind size is critical. It balances the recovery gained from better liberation against the increased cost and problems caused by over-grinding.
Crush the ore in stages. Large run-of-mine ore first goes to a primary Jaw Crusher. The product from the jaw crusher then goes to secondary and sometimes tertiary Cone Crushers. Using stages is more energy-efficient than trying to achieve a large size reduction in one machine. The final crushed product size is typically around 10-20 mm. This size is suitable for feeding the grinding mills. ZONEDING can provide a range of Crushing Equipment for these stages.
After crushing, the ore goes to grinding mills. Ball Mills are very common. They use steel balls to grind the ore in a slurry. Rod Mills use steel rods. They are sometimes used for primary grinding because they produce fewer ultra-fine particles compared to ball mills. This can be good for ores where slime generation is a major concern. The grinding circuit often works in closed loop with classifiers like Hydrocyclones or Spiral Classifiers. These separate particles by size. They send coarse particles back to the mill for more grinding. They send fine enough particles to the next stage (flotation).
Recover galena first. Use specific collectors like xanthates. Keep the pH neutral or slightly alkaline (7.5-8.5). Add chemicals like zinc sulfate and sulfites to keep zinc and pyrite from floating.


Differential flotation is the most common method for lead-zinc ores. This means that need to float one mineral type first, then the other. Usually, float the lead mineral (galena) first. This is called the lead rougher flotation stage.
Galena usually floats well in neutral or slightly alkaline conditions. Need to adjust the pH to around 7.5 to 8.5. Might use soda ash (sodium carbonate) or sometimes a small amount of lime for pH control. The main chemicals added are collectors and frothers. Collectors make the galena surface water-repellent (hydrophobic) so it attaches to air bubbles. Xanthates (like Sodium Isobutyl Xanthate – SIBX or Potassium Amyl Xanthate – PAX) are common collectors for galena. Frothers (like MIBC or pine oil) create stable bubbles to carry the mineral particles to the surface.
While wanting galena to float, need to prevent sphalerite (zinc mineral) and pyrite (iron sulfide) from floating at the same time. Add depressants (or suppressants) to make these minerals water-loving (hydrophilic). Zinc sulfate (ZnSO₄) is commonly used to depress sphalerite. Sulfites (like sodium sulfite Na₂SO₃ or sodium metabisulfite SMBS) can help depress both sphalerite and pyrite. In the past, sodium cyanide (NaCN) was often used, especially for pyrite depression. But due to its toxicity, mines try to avoid it or use alternatives now. Getting the type and amount of depressants right is key for a clean lead concentrate.
So, a typical reagent combination for lead flotation might include:
Generally use equipment like Mixer tanks to ensure reagents mix well before flotation in Flotation Machines Precise control is needed.
After lead flotation, add copper sulfate (CuSO₄). This activates the sphalerite surface. Then, raise the pH to 10-11.5 with lime. This keeps any remaining pyrite down. Finally, add collectors like xanthates to float the zinc.



Once the lead minerals have been floated off, the slurry (tailings from the lead circuit) goes to the zinc flotation circuit. The main goal here is to recover the sphalerite (ZnS). Sphalerite usually needs help to float after being depressed in the lead circuit.
The key step is activation. Add copper sulfate (CuSO₄). Copper ions replace zinc ions on the sphalerite surface. This creates a surface similar to copper sulfide minerals, which are easily floated by xanthate collectors. The amount of copper sulfate needed depends on the amount of sphalerite and other factors. Adding too little results in poor zinc recovery. Adding too much is wasteful and can sometimes activate other unwanted minerals like pyrite. Careful control is essential. The activation process needs some time, so copper sulfate is usually added to a conditioning tank (Mixer tanks) before the zinc flotation cells.
Adjust the pH in the zinc circuit. Typically, raise the pH significantly, often to between 10 and 11.5. Lime (calcium hydroxide, Ca(OH)₂) is almost always used for this. The high pH serves two main purposes. First, it helps to keep any remaining pyrite depressed. Pyrite flotation is strongly suppressed at high pH. Second, this pH range is generally favorable for the flotation of copper-activated sphalerite using xanthate collectors. However, as mentioned before, too much lime (very high pH) can start to depress the sphalerite itself, even after activation. Finding the optimal pH is crucial.
With the sphalerite activated and pyrite depressed, need to add a collector, usually a xanthate (like SIBX or a stronger one like PAX), and a frother. The activated sphalerite particles attach to the air bubbles generated in the Flotation Machines and are collected as a zinc concentrate. Like the lead circuit, the zinc circuit often includes rougher, scavenger, and cleaner stages to maximize recovery and grade.
Precise control means adding the exact right amount of collectors, frothers, depressants, activators, and pH regulators. They must be added at the right places and given enough time to work. This ensures only the target mineral attaches to bubbles.
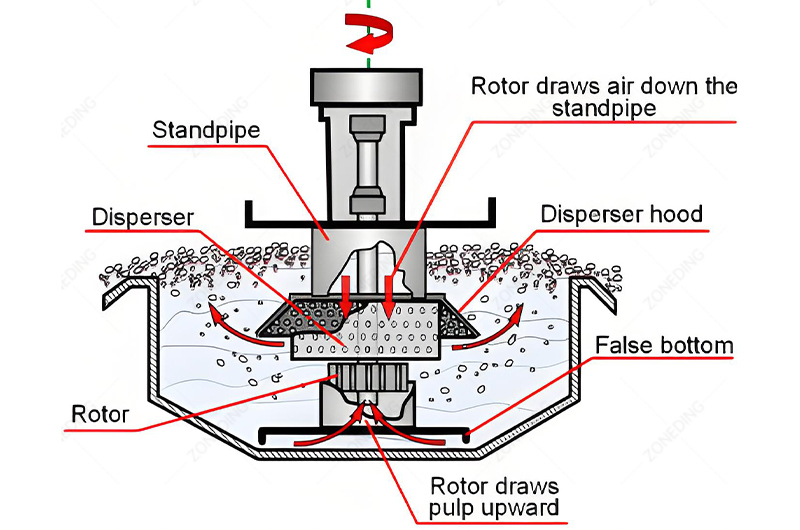
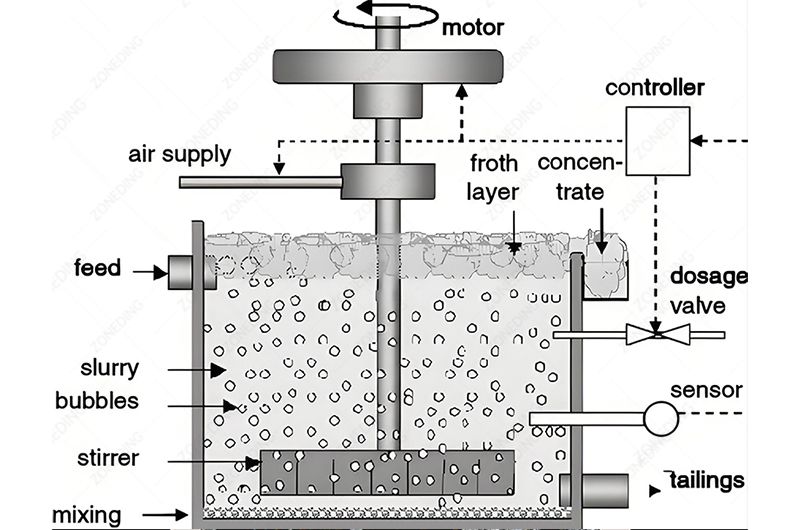
Differential flotation of lead and zinc is often described as an “art” as much as a science. Getting clean separation relies heavily on managing the complex chemistry in the flotation pulp. Precise control of reagents is the absolute key.
It’s not just what chemicals you add, but how much, where, and when. Each reagent needs a specific concentration to work effectively. Under-dosing leads to poor recovery or poor depression. Over-dosing wastes expensive chemicals and can cause unintended effects, like depressing the valuable mineral or floating unwanted gangue. Reagents also need time to work. Depressants need time to adsorb onto the minerals they are supposed to suppress before the collector is added. Activators need time to change the surface properties. Reagents are typically added at specific points: to the grinding mill, to conditioning tanks (Mixer) before flotation, or sometimes directly into flotation cells. The flow rate of the pulp through the circuit must allow sufficient conditioning time.
The quality of the process water is very important. Dissolved ions in the water can react with flotation reagents or mineral surfaces. For example, calcium and magnesium ions (water hardness) can precipitate some collectors, reducing their effectiveness. Certain metal ions can accidentally activate or depress minerals. In plants that recycle process water, these ions can build up over time, making flotation control more challenging. Regular water quality monitoring is necessary. Sometimes water treatment is needed.
While automation helps maintain stability, experienced operators are invaluable. They monitor the appearance of the froth in the flotation cells. The froth’s color, bubble size, and texture give clues about the flotation performance. Skilled operators can make small adjustments to reagent dosages or pH in real-time based on these observations and regular sampling results. They adapt to minor changes in the ore feed, which automated systems might struggle with. This ability to fine-tune the delicate chemical balance is often what separates a highly efficient operation from an average one.
Suppress pyrite mainly by controlling pH. High pH (over 10 using lime) in the zinc circuit helps a lot. Also use chemical depressants like sulfites or cyanide alternatives during lead flotation to keep pyrite from floating.


Pyrite (FeS₂) is often the most common sulfide mineral found alongside galena and sphalerite. Unfortunately, it tends to float relatively easily with common sulfide collectors like xanthates. If too much pyrite floats into the lead or zinc concentrate, it dilutes the grade (lowers the percentage of Pb or Zn). This reduces the concentrate’s value. Smelters also have limits on the iron (Fe) content derived from pyrite. So, effective pyrite suppression is essential for producing marketable concentrates.
Besides diluting the concentrate grade, pyrite can consume reagents meant for lead and zinc. It increases the total amount of material that needs to be handled and processed. In some cases, if the tailings containing pyrite are exposed to air and water, they can cause acid rock drainage (ARD), which is a serious environmental issue. Therefore, keeping pyrite out of the concentrates and managing it in the tailings is important for both economic and environmental reasons.
One of the main tools for pyrite suppression, especially in the zinc circuit, is high pH. As mentioned before, lime is added to the zinc circuit to raise the pH, often above 10 or 11. In this highly alkaline environment, pyrite surfaces tend to become hydrophilic (water-loving), making it much harder for collectors to attach and for the pyrite to float. This is a very effective way to suppress pyrite during zinc flotation. In the lead circuit, the pH is usually lower (around 8), which is not high enough to fully suppress pyrite on its own, so chemical depressants are more critical there.
In the lead circuit (and sometimes to help in the zinc circuit), chemical depressants are used. As mentioned, sodium cyanide was historically effective but is often avoided now. Alternatives include various sulfite-based chemicals (like Na₂SO₃ or NaHSO₃ or SMBS) or specific organic depressants. These chemicals adsorb onto the pyrite surface, preventing the collector from attaching. The choice of depressant and its dosage needs careful testing and control, as some depressants can also affect galena or sphalerite if not used correctly. Some types of pyrite are naturally more floatable or harder to depress than others, adding to the challenge.
For fine ores, use stage grinding/flotation, desliming with a Hydrocyclone, special reagents, or carrier flotation. For oxidized ores, try sulfidization then flotation, acid leaching, or gravity methods. Complex ores often need a combination of techniques.

While standard differential flotation works well for many lead-zinc sulfide ores, some ores present significant challenges. These include ores where the minerals are locked together at a very fine size, ores that have been exposed to weather and become oxidized, or ores that contain a complex mix of minerals.
When galena and sphalerite are very finely disseminated, the ore must be ground extremely fine (e.g., below 20 microns) for liberation. This creates a lot of slime particles. Slimes cause problems: high reagent consumption, poor flotation selectivity, and mechanical carry-over of gangue into the concentrate. Strategies include:
Oxidized lead minerals (like cerussite PbCO₃, anglesite PbSO₄) and zinc minerals (like smithsonite ZnCO₃, hemimorphite Zn₄Si₂O₇(OH)₂·H₂O) do not respond well to standard sulfide flotation methods. Options include:
Ores containing both sulfide and oxide minerals (mixed ores) are particularly challenging. They might require separate circuits for sulfide and oxide flotation, or complex reagent schemes trying to recover both types. Ores with many different valuable or penalty elements (e.g., high arsenic, antimony, bismuth) also require careful process design and reagent selection to achieve selective separation. Thorough lab and pilot testing is absolutely essential for these difficult ores.
Silver (Ag) usually goes with the lead into the lead concentrate. Copper (Cu) minerals might float with the lead or need a separate step. Cadmium (Cd) follows the zinc into the zinc concentrate. It cannot be separated easily and must be managed.


Lead-zinc ores often contain other metals that can be economically important or environmentally significant. The main ones are typically silver (Ag), copper (Cu), and cadmium (Cd). Gold (Au) can also be present sometimes. How these are recovered (or managed) depends on how they occur in the ore.
Silver is very often associated with galena (PbS). It can substitute for lead within the galena crystal structure or occur as tiny inclusions of separate silver minerals (like argentite, Ag₂S) within or attached to the galena. Because of this close association, most of the silver follows the lead during flotation. Therefore, maximizing lead recovery in the lead circuit is usually the best way to maximize silver recovery. The silver content significantly increases the value of the lead concentrate. Smelters pay for the silver in the lead concentrate, usually above a certain minimum grade. So, managing silver recovery is an important economic consideration.
Cadmium (Cd) behaves differently. It is chemically very similar to zinc. Cadmium almost always substitutes for zinc within the sphalerite (ZnS) crystal structure. Because it’s part of the sphalerite mineral itself, physical separation methods like flotation cannot separate cadmium from zinc. As a result, nearly all the cadmium in the ore reports to the final zinc concentrate. This is important because cadmium is a toxic heavy metal. Zinc smelters have very strict limits on the maximum allowable cadmium content in the zinc concentrates they buy (often below 0.2% Cd). High cadmium levels lead to penalties or even rejection of the concentrate. There’s no easy way to remove cadmium during concentration. Mines must know their cadmium levels early on and manage the zinc concentrate grade to try and stay within smelter limits. Cadmium content is a critical factor in the marketability and value of zinc concentrate.
If the ore contains significant amounts of copper minerals, such as chalcopyrite (CuFeS₂), the processing route might need to be adjusted. Chalcopyrite sometimes floats along with galena in the lead circuit. If copper levels are high enough, a separate copper flotation stage might be added, often before the lead flotation (as a copper-lead bulk concentrate followed by separation) or sometimes after the zinc flotation. The decision depends on the amount and type of copper minerals present. ZONEDING can help design circuits that incorporate copper recovery if needed.
Smelters need minimum levels of lead (Pb) in lead concentrate and zinc (Zn) in zinc concentrate. They also set maximum limits for harmful impurities like cadmium (Cd), arsenic (As), antimony (Sb), bismuth (Bi), and others.


The lead and zinc concentrates produced by the processing plant are intermediate products. They are sold to smelters, which extract the final pure metals. Smelters have specific requirements for the feed materials they can process efficiently and safely. These requirements are set out in concentrate purchasing contracts. Meeting these specifications is critical for the mine’s revenue.
Smelters need a certain minimum percentage of the main metal to operate economically.
Just as important as the main metal grade are the levels of impurities. Certain elements interfere with the smelting process, affect the quality of the final metal, or cause environmental problems. Smelters set strict maximum limits for these. Common penalty elements include:
Exceeding these limits often results in financial penalties charged by the smelter. Very high levels can lead to the concentrate being rejected altogether. Knowing these limits guides the targets for the concentration process.
Concentrates are usually dewatered before shipping, but smelters also have maximum moisture content limits (e.g., 8-10%). Excessive moisture adds weight (increasing transport costs) and can cause handling problems (like freezing in cold climates). The particle size distribution might also be specified sometimes. Producing concentrates that consistently meet smelter specifications requires good process control in the plant.
A modern plant needs Crushing Equipment (like Jaw Crushers, Cone Crushers), grinding mills (Ball Mill), classifiers (Hydrocyclone, Spiral Classifier), Flotation Machines, thickeners (High Efficiency Concentrator), filters, and material handling systems (Vibrating Feeder, conveyors, pumps).
A typical lead-zinc differential flotation plant uses several types of equipment working together in a sequence.
As a manufacturer, ZONEDING supplies many of these core components. The main stages and equipment involved are:
Improve the process by better grinding control (avoid over-grinding), fine-tuning reagent amounts using analyzers, saving and recycling water, using energy-efficient machines, and using more automation for stable control.


Optimizing a lead-zinc plant aims to lower operating costs (OPEX) and maximize revenue by improving metal recovery and concentrate quality. There are several areas where improvements can often be made.
Crushing and especially grinding consume a large amount of electricity, often the biggest single operating cost.
Flotation reagents are another major cost.
Making improvements in these areas can lead to significant cost savings and increased profitability over the life of the mine.
Key things are building and managing secure tailings dams, treating process water to remove heavy metals (Pb, Zn, Cd) and chemicals before release or reuse, and controlling dust emissions.
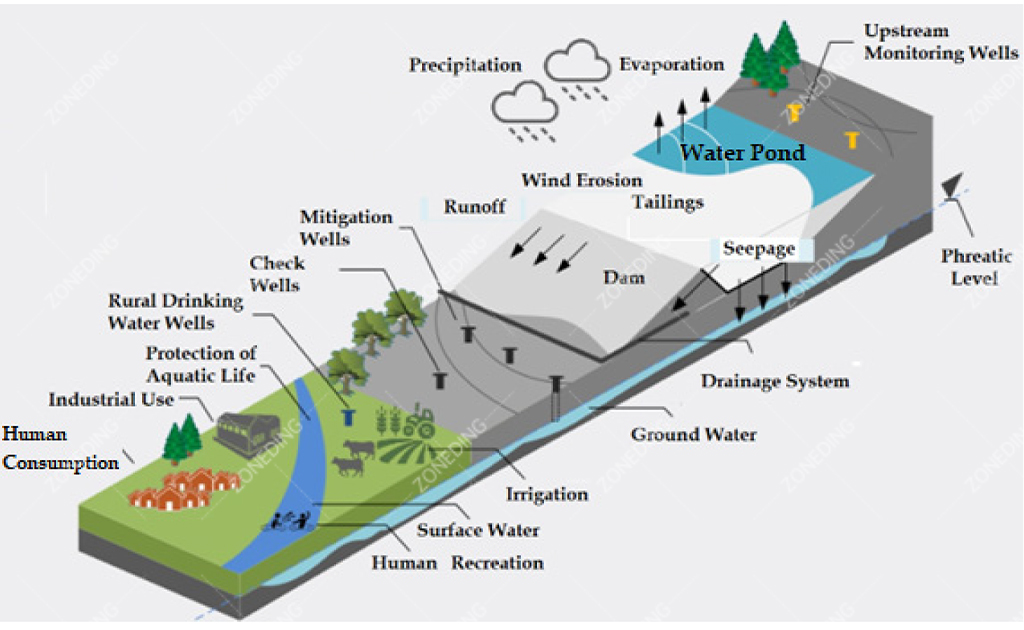
Modern mining operations must prioritize environmental protection. Lead-zinc processing generates waste streams, primarily tailings (the leftover ground rock after valuable minerals are removed) and process water, which need careful management.
Tailings are typically pumped as a slurry to a specially engineered storage facility (tailings dam).
Process water comes into contact with minerals and reagents. Before being discharged to the environment (if allowed) or recycled back into the process, it often needs treatment.
Responsible environmental management is not just a legal requirement but also essential for maintaining a company’s social license to operate.
Success relies on understanding ore, precise chemical control, managing byproducts, and responsible waste handling for profit and environmental care.
We use cookies to ensure that we give you the best experience on our website. If you continue to use this site we will assume that you are happy with it.
Privacy Policy