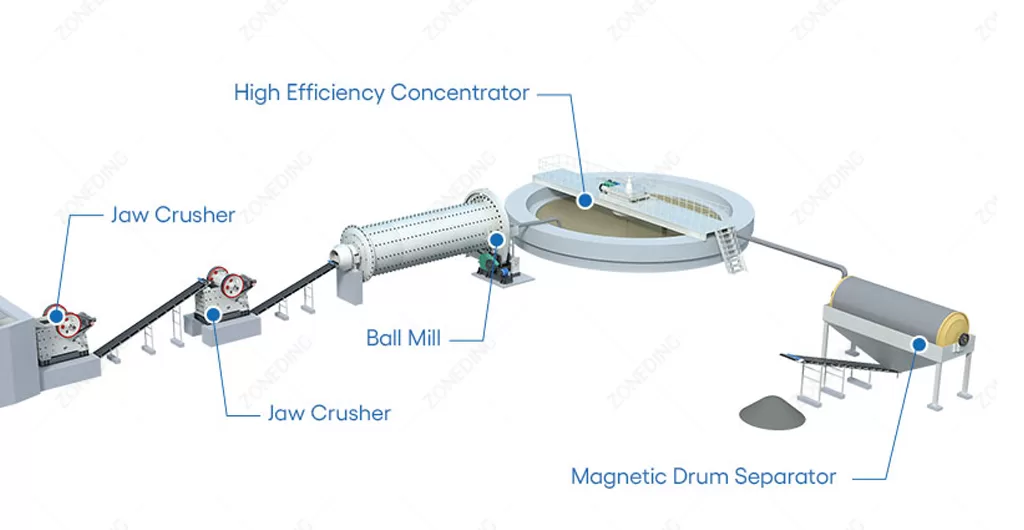
Gold Ore Beneficiation Plant: Essential Machinery & Systems
3814Get a complete list of essential equipment required for a successful gold ore beneficiation plant. Understand the machinery needed for efficient gold recovery.
View detailsSearch the whole station Crushing Equipment
Raw kaolin contains iron impurities that stain the clay, reducing its brightness and commercial value. These impurities severely limit its use in high-value applications like paper coating and premium ceramics, directly impacting profitability for producers.


The most effective method for kaolin purification is a multi-stage process combining physical separation, primarily high-intensity magnetic separation, with chemical bleaching. The specific combination of these processes is tailored to the types and amounts of iron impurities present in the raw ore.
The journey from a low-value raw clay to a high-purity industrial mineral is a precise, multi-step operation. A successful purification circuit is not built on a single piece of equipment but on a synergistic flowsheet where each stage prepares the material for the next. This approach maximizes iron removal while controlling operational costs.
Kaolin is a common industrial mineral, but its value is entirely dependent on its purity. Understanding its primary applications is key to understanding why the removal of iron impurities is so critically important for producers.
Kaolin is a hydrated aluminum silicate clay mineral. It is a vital raw material in high-value industries including paper, ceramics, paints, and plastics due to its natural whiteness, chemical stability, and fine particle size. Its performance in these applications depends heavily on its brightness.
The commercial value of kaolin is directly correlated with its whiteness, or brightness. Iron is the primary coloring impurity that reduces brightness. Therefore, the entire goal of kaolin processing is to remove iron and other discoloring elements to meet the stringent specifications of different industries.




Different markets demand different levels of purity, which dictates the complexity of the required processing flowsheet.
| Industry Application | Key Required Properties | Impact of Iron Impurities |
|---|---|---|
| Paper Manufacturing | High brightness (>88%), fine particle size, controlled viscosity. | Causes a yellow or grey tint, reducing paper quality and brightness. |
| Ceramics & Sanitaryware | High plasticity, controlled firing color, high strength. | Causes unwanted color spots and defects after firing. Reduces whiteness. |
| Paints & Coatings | High opacity, chemical inertness, good dispersion. | Reduces whiteness and hiding power, affecting the final color of the paint. |
| Rubber & Plastics | Reinforcing filler, improves tensile strength, inertness. | Acts as an abrasive impurity, can affect vulcanization process. |
Iron impurities in kaolin are not a single entity. Using the wrong purification process for the type of iron present is like using the wrong tool for a job—it is ineffective, inefficient, and costly. A successful process begins with identifying the enemy.
The process choice depends on the form of iron. High-intensity magnetic separation removes discrete iron oxide particles. Flotation targets iron-bearing accessory minerals like mica. Chemical bleaching dissolves ultra-fine surface stains and refractory iron oxides.
Iron exists in three distinct forms within a kaolin deposit. Each form requires a specific removal strategy. A thorough mineralogical analysis of the raw ore is the critical first step before designing any purification plant.

Magnetic separation is a cornerstone of modern kaolin processing. However, it is a specialized tool with specific capabilities and limitations. Relying on it as a sole solution can lead to disappointing results and a failure to meet product quality targets.
A high-intensity Magnetic Separator is the best choice for removing weakly magnetic particulate iron oxides. It is highly effective for this task but cannot remove non-magnetic impurities, ultra-fine surface stains, or structural iron.
Wet High-Intensity Magnetic Separators (WHIMS) are the industry standard. They work by passing a kaolin slurry through a matrix-filled canister placed within a powerful magnetic field. The matrix creates high-gradient zones that capture weakly magnetic iron-bearing particles.
While powerful, the WHIMS unit must be integrated correctly into the flowsheet to be effective. Its performance depends on proper feed preparation and operational control. The matrix inside the separator is very fine and can be easily clogged by oversized particles. Therefore, meticulous pre-screening and de-gritting of the kaolin slurry using equipment like Hydrocyclones is absolutely essential before the slurry reaches the magnetic separator. Furthermore, the cleaning cycle of the separator, which flushes the captured magnetics, must be optimized. Setting this cycle dynamically based on the iron content of the feed, rather than a fixed timer, prevents saturation of the matrix and ensures consistent product quality.
Chemical bleaching is the final step used to achieve the highest levels of brightness. However, the high cost of chemical reagents and the need to manage wastewater can make this stage a major operational challenge if not engineered and controlled correctly.
Costs are managed through strict process optimization: precise pH control, using de-aerated water to prevent reagent waste, and optimizing retention time. Environmental impacts are solved by implementing a robust wastewater treatment circuit to neutralize pH and remove dissolved solids before discharge.
The most common method is reductive bleaching using sodium dithionite. This chemical converts insoluble, colored ferric iron (Fe³⁺) into soluble, nearly colorless ferrous iron (Fe²⁺), effectively dissolving the stain off the kaolin particles.
Three factors are critical for an efficient and cost-effective bleaching stage.
Sending raw ore directly to the advanced magnetic and chemical stages can be highly inefficient. Cost-effective pre-treatment steps are designed to remove bulk impurities, which reduces the load on the more expensive downstream equipment and lowers overall processing costs.
Yes, pre-treatment is often required. Froth flotation is used to remove iron-bearing accessory minerals like mica and tourmaline. Gravity separation, using equipment like Spiral Classifiers, is used to remove coarse sand (quartz) and other heavy minerals.
The decision to include these pre-treatment steps is based entirely on the mineralogy of the raw ore. If the ore analysis shows a high content of sandy quartz, a de-gritting stage is non-negotiable to protect downstream equipment. If iron-bearing micas are present, a flotation circuit is essential because these minerals are often not magnetic enough to be fully removed by a WHIMS but will still cause discoloration. Adding these relatively low-cost stages upfront significantly improves the efficiency and reduces the operating cost of the more expensive final purification stages. It is a critical step in designing an economically viable plant.
A “one-size-fits-all” flowsheet does not exist in kaolin processing. An efficient and profitable plant is the result of a carefully designed process that is precisely matched to the unique characteristics of the raw ore and the strict quality demands of the target market.
A custom process is designed by first conducting a detailed mineralogical analysis of the ore to identify the iron forms and particle size. Then, a multi-stage flowsheet is engineered to meet the specific brightness and chemical specifications required for the intended market (e.g., filler grade vs. coating grade).
The design process is a strategic balancing act. The goal is to achieve the target product quality at the lowest possible capital and operating cost.
The complexity of the flowsheet increases directly with the required final brightness of the product.
| Target Market Grade | Typical Brightness | Key Processes Required |
|---|---|---|
| Filler Grade | 80-84% | Slurrying, Degritting, Particle Size Classification |
| Ceramic Grade | 84-88% | All of the above + High-Intensity Magnetic Separation |
| Coating Grade | 88-92%+ | All of the above + Selective Flocculation, Chemical Bleaching |
Building a reliable and efficient kaolin production line requires selecting the right core equipment for each stage of the process. Each machine has a specific function, from creating the initial slurry to drying the final, high-purity product.
A complete kaolin line includes equipment for slurry preparation (blungers), classification (Hydrocyclones), physical purification (Wet High-Intensity Magnetic Separator), chemical purification (leaching tanks), dewatering (filter presses), and drying (Rotary Dryers).
The selection and sizing of this equipment depend on the plant’s target capacity, ore characteristics, and final product specifications.




| Process Stage | Core Equipment | Primary Function |
|---|---|---|
| Slurrying & Degritting | Blunger, Agitation Tank, Spiral Classifier | Creates a pumpable slurry and removes coarse sand. |
| Classification | Hydrocyclone, Centrifuge | Separates kaolin into specific fine particle size fractions. |
| Physical Purification | High-Intensity Magnetic Separator, Flotation Machine | Removes magnetic minerals and other accessory mineral impurities. |
| Chemical Purification | Leaching Tanks, Mixers | Dissolves remaining iron oxide stains to increase brightness. |
| Dewatering & Drying | High Efficiency Concentrator, Filter Press, Rotary Dryer | Removes water to produce the final powdered kaolin product. |
Successful kaolin purification hinges on a deep understanding of the ore’s unique mineralogy. An effective and profitable process is always a multi-stage approach, tailored specifically to combat the different forms of iron impurities present in the feed material.
Get a complete list of essential equipment required for a successful gold ore beneficiation plant. Understand the machinery needed for efficient gold recovery.
View detailsThe perfect solution for gold mining. Our portable hard rock crushers are engineered to process gold-bearing ore efficiently.
View detailsEfficient stone processing requires a well-planned Stone Crushing Flow to maximize output and minimize costs. Modern mining operations in 2026 prioritize automation and energy-saving designs. A successful production line relies on the c...
View detailsDeciding between a ball mill and a rod mill? This guide breaks down the selection criteria, from feed size to desired output, for your grinding circuit.
View details